
DON'T PANIC! You will need to not totally panic when shown something like this. You don't need to have the understanding that a chemist would have, but these types of molecular diagrams should not want to make you gnaw off your leg to get away! Therefore... (don't memorize this stuff -- just stare at it until you lose your fear and have some basic understanding of what you're looking at -- btw, it's the molecular structure of caffeine)...
Some Basics About Molecules

I. Atoms Are the Basic Building Blocks of Matter

- atoms are built from
- protons (in the nucleus)
- neutrons (in the nucleus)
- electrons (that orbit around the nucleus)
- molecules are made of atoms that bond together
- atoms bond together when their electron clouds interact, and electrons from one atom fill in holes in the electron clouds of other atoms
- in organic molecules, the electrons are usually (but not always) shared fairly equally between the atoms - a "nonpolar bond" creating a "nonpolar molecule" (yes, also called a "covalent bond" for those of you who've had a course in chemistry)
- electrons are usually shared in pairs (a "single bond"), but sometimes two pairs of electrons are shared, creating a "double bond"
- organic matter (tissue) is made up mostly of four atoms
- hydrogen - has one "hole" so forms one bond
- carbon - has four "holes" so forms four bonds
- nitrogen - has three "holes" so forms three bonds
- oxygen - has two "holes" so forms two bonds
- the "tinker toy" model of atoms - is illustrated below

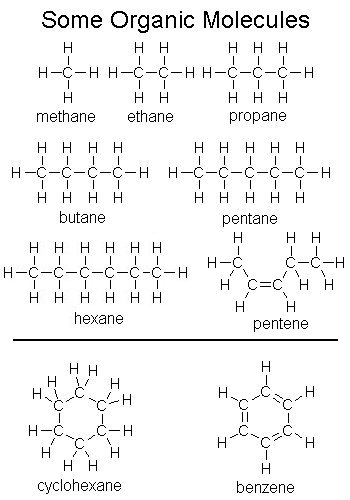
II. Carbon-Carbon Bonds are Very Important In Living Tissue
- all life on earth is carbon-based
- life is made from molecules that have long carbon chains and carbon rings
- hydrocarbons are molecules comprised entirely of carbon and hydrogen - carbon-hydrogen bonds are nonpolar
- carbon-carbon bonds are obviously nonpolar, since the two carbon atoms are identical and share electrons equally
- therefore, electric charge in hydrocarbons is pretty equally distributed throughout the molecule and, therefore, the molecule exhibits little external electrical properties
- O−H and N−H bonds, however, are polar because the oxygen and nitrogen atoms pull harder on the shared electrons than the hydrogens do - molecules with these bonds are "polar" and do exhibit external electrical properties
- there are so many carbon-hydrogen bonds (and atoms) to draw in most organic molecules that
often only a skeleton molecule is drawn in which the carbons and hydrogens bonded to them
are left out, and the presence of a carbon is indicated by bend


- carbon rings, like benzene, are especially likely to be represented this way

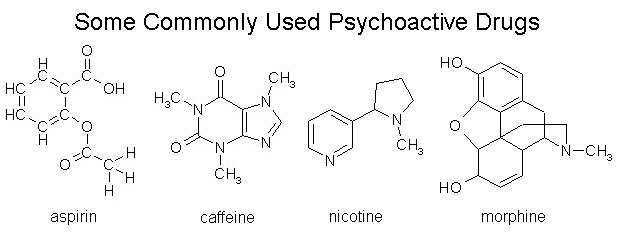
IV. Some Common Kinds of Organic Molecules
- acids

- amines (these ones are all "monoamines")

- amino acids

- fatty acids (palmitic acid and arachidonic acid shown)


- peptides and proteins


- enzymes
- receptor sites, e.g., for neurotransmitters
- ion channels
- ion and neurotransmitter transporters
- some neurotransmitters and hormones are peptides
- alcohols (some of which, as you've probably heard, are psychoactive!)

- sugars, saccharides, and carbohydrates (C−OH2)


- aldehydes, ketones, esters

IV. So What? (A reasonable question!)
|
Now you have to start paying attention, because now it's becoming... PSYCHOPHARMACOLOGY! |

- If you looked at those molecules and said to yourself, "Hey! Those molecules are all very similar," then you're getting it. The other thing you should have noticed is the presence of −OH and −NH groups on some of the molecules, which will tend to make them polar.
- molecules with similar chemical structure tend to behave similarly in the brain
- molecules that are nonpolar dissolve in fat better and, therefore, cross the blood-brain barrier more easily
- some lessons in metabolism - the process by which molecules are built and destroyed in
the body
- enzymes - protein molecules that act as organic catalysts
- a catalyst speeds up the rate at which a reaction goes
- organic reactions would be very slow or not go at all without catalysts (enzymes)
- each reaction has its own enzyme (some enzymes operate more generally than this)
- when the name of the substance ends in -ase, it's an enzyme
- example: MAO or monoamine oxidase

- enzyme activity can be regulated
- the role of enzymes in neurotransmitter biosynthesis

- some lessons in alcohol metabolism
- ethanol is broken down in the body to acetaldehyde

- this is partially responsible for the "hangover" the following morning
- but it could be worse...
- methanol is broken down to... what is that stuff?

- and isopropanol is broken down to... ?

- kinda gives a whole new meaning to the expression "getting pickled"!!!
Return to PSYC 460 Main Page
- ethanol is broken down in the body to acetaldehyde
- enzymes - protein molecules that act as organic catalysts